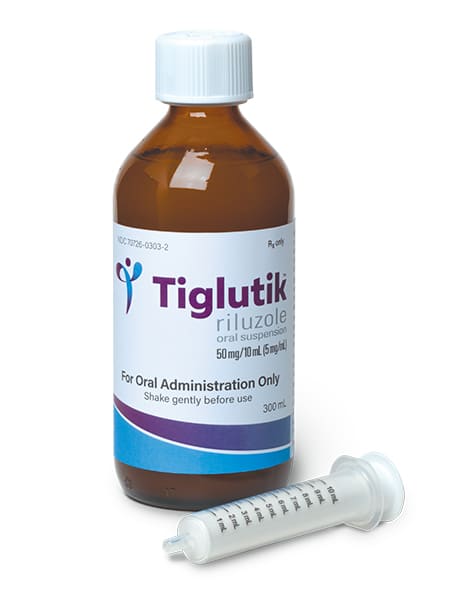
TIGLUTIK is the only formulation of riluzole approved by the FDA for both oral and PEG tube use. One dose of TIGLUTIK 50 mg (10 mL) (2 teaspoons) is equivalent to one RILUTEK® (riluzole) 50 mg tablet and it is taken twice a day, every 12 hours. TIGLUTIK has a mildly thick consistency and developed to be easy to swallow or use with a PEG tube. Finally, patients no longer have to crush riluzole tablets since TIGLUTIK meets the needs of all people with ALS. Patients should discuss with their physician starting TIGLUTIK as soon as possible.
TIGLUTIK was created by scientific experts to flow smoothly using advanced drug suspension technology. Based on the standards developed by the International Dysphagia Diet Standardization Initiative (IDDSI), TIGLUTIK has a mildly thick consistency (IDDSI criteria Level 2), which is essential for people with ALS who have difficulty swallowing because thin liquids are difficult to take.